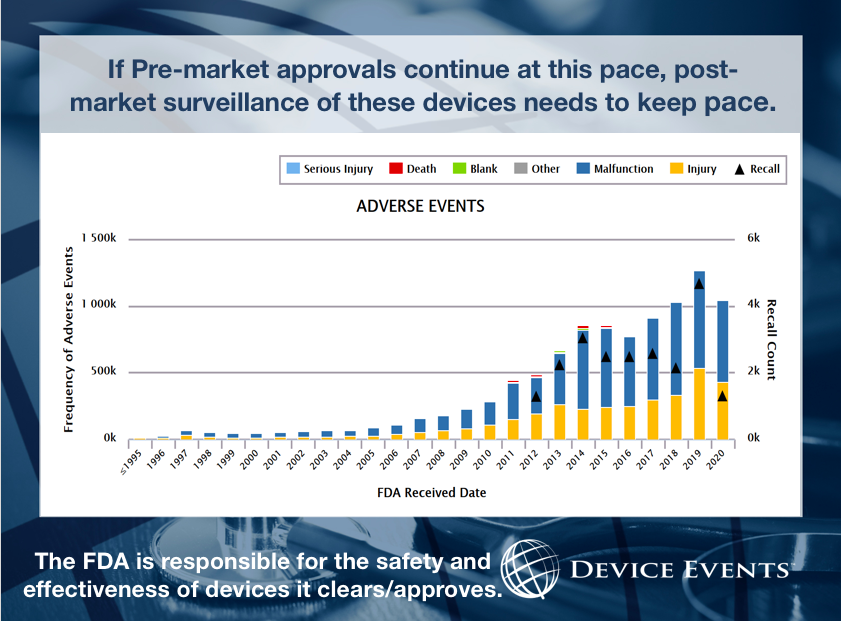
Medical Device Safety
Working for improved FDA oversight of medical devices, including tracking harm caused by devices
COMMITTEE:
- Medical Device Roundtable
- Leaders: Linda Radach, Lisa McGiffert
ACTIONS:
- PSAN member Madris Kindard quoted in article about Senators requesting the Government Accounting Office, a Congressional Watchdog, to investigate the FDA’s Oversight of Medical Device Recalls (Jan 2024)
- PSAN endorses the Medical Device Recall Improvement Act by US Senators Durbin and Schakowsky to bolster communication between medical device manufacturers, hospitals and health care professionals, and patients (Sept 2023)
- PSAN member Madris Kinard’s presentation to the FDA Patient Engagement Advisory Committee on Equity (Sept 2023)
- Patient, Consumer, and Public Health Coalition letter to FDA Commissioner Califf expressing concern about FDA ignoring their own scientists when making approval decisions and failing to value the independent expertise of Advisory Committee members. (July 2023)
- Patient advocate Maria Gmitro, Breast Implant Safety Alliance & member of PSAN Medical Device Roundtable, speaks at FDA hearing on medical device safety (2022)
- Lisa McGiffert with Patient Safety Action Network, joins Dr. Larry Muscarella and Justin Poulin to share her passion for patient advocacy and ending harm by increasing transparency and accountability on this episode of Transmission Control (2022)
- PSAN member Madris Kinard’s presentation with recommendations for recall communications (Oct 2021)
- PSAN Medical Device Roundtable comment to FDA about adverse immunological to metals in medical device implants (Dec 2019)
RESOURCES:
